A biocompatible polymer may assist ship vaccines and medicines with lowered threat of the uncommon harmful adversarial response known as anaphylaxis. Researchers on the Nationwide Institute of Superior Industrial Science and Know-how (AIST) in Japan have developed the polymer and carried out preliminary checks, which they report within the journal Science and Know-how of Superior Supplies.
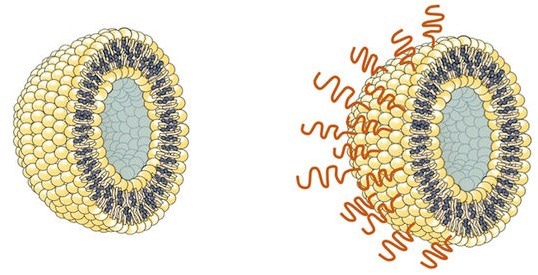
Till now, the polymer of selection for encasing and delivering vaccines has been poly(ethylene glycol) (PEG). This artificial, versatile, water-soluble materials has been used to encompass some COVID-19 vaccines carried throughout the tiny spherical packages generally known as liposomes.
Sadly, some recipients have suffered an anaphylactic response to PEG, through which the immune system mounts an allergic response to the overseas materials. Signs of anaphylaxis vary from minor pores and skin irritations, to respiratory issue, nausea and, within the worst circumstances, unconsciousness and sudden loss of life.
The choice polymer is a type of fatty biomolecule known as a lipid, and is conjugated to 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer.
This new substance spontaneously binds to the surface of liposome particles when blended with them in water. Crucially, the polymer just isn’t acknowledged by the antibodies that the physique can generate in response to PEG, and checks counsel it doesn’t stimulate some other antibodies that might trigger an allergic response. This could enable coated liposomes containing a vaccine to be retained within the physique for an extended time with out being cleared by the immune system, along with avoiding anaphylaxis.
“We’ve got additionally discovered that the polymer avoids different interactions with proteins within the blood which may in any other case intervene with its results, and it additionally prevents liposomes from aggregating collectively,” says molecular engineer Yuji Teramura of the AIST workforce.
Exams verify the coated liposomes can stay steady in storage for 14 days, ample for actual medical purposes.
“All of the indications counsel that our expertise must be appropriate for delivering vaccines into sufferers who develop anaphylaxis in response to PEG,” Teramura concludes.
The polymer should now be totally examined in varied actual vaccine purposes. The workforce is transferring into this subsequent essential part of the event course of, previous to eventual medical trials in people.
Supplied the animal and subsequent medical trials go properly, the expertise ought to supply alternatives for delivering medicine into the physique, along with vaccines. Supply programs equivalent to liposomes are generally wanted to guard medicine from biochemical processes which may degrade them. This could make sure that they attain the goal illness tissues whereas remaining of their energetic kind.
Supply: https://www.tandfonline.com/STAM