When Srikanth Singamaneni and Man Genin, each professors of mechanical engineering and supplies science on the McKelvey College of Engineering at Washington College in St. Louis, established a brand new collaboration with researchers from the College of Medication in late 2019, they did not know the panorama of infectious illness analysis was about to shift dramatically. In a convention room overlooking Forest Park on a lovely fall day, the crew had one purpose in thoughts: sort out the most important infectious illness drawback going through the world proper then.
“Srikanth and I had a imaginative and prescient of a easy, quantitative diagnostic software, so we related with infectious illness physicians right here at WashU and requested them, ‘What are crucial questions that may very well be answered in the event you may get actually detailed data cheaply on the level of care?'” stated Genin, the Harold and Kathleen Faught Professor of Mechanical Engineering.
“Greg Storch instructed us that one of the crucial vital challenges going through the sphere of infectious illness is discovering a approach to determine rapidly if a affected person has a bacterial an infection and may get antibiotics or has a viral an infection, for which antibiotics is not going to be efficient.”
Storch, MD, the Ruth L. Siteman Professor of Pediatrics on the College of Medication, was interested by ailments that have an effect on most individuals repeatedly—colds, strep throat or the flu—however that weren’t getting as a lot analysis consideration as rarer ailments. “Even with nice advances which were made in infectious illness diagnostics, there may be nonetheless a distinct segment for checks which can be easy, speedy and delicate,” Storch stated. “It might be particularly highly effective if they may present quantitative data. Exams with these traits may very well be employed in refined laboratories or within the subject.”
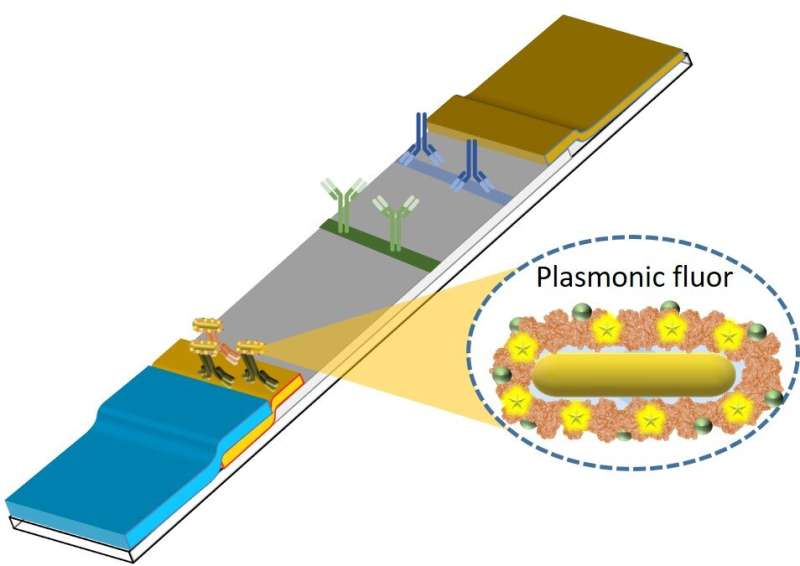
Drawing on his years of expertise in creating nanomaterials for functions in biology and medication, Singamaneni, the Lilyan & E. Lisle Hughes Professor, sought to beat these limitations in point-of-care diagnostic checks. Singamaneni and his lab developed ultrabright fluorescent nanolabels referred to as plasmonic-fluors, which may very well be rapidly built-in into a typical testing platform, the lateral movement assay (LFA).
Plasmon-enhanced LFAs (p-LFAs) enhance cheap, available speedy checks to ranges of sensitivity required by physicians for confidence in check outcomes with out the necessity for lab-based affirmation.
Based on findings revealed Feb. 2 in Nature Biomedical Engineering, the crew’s p-LFAs are 1,000 occasions extra delicate than typical LFAs, which present outcomes through a visible colour and fluorescence sign on the strip. When analyzed utilizing a fluorescence scanner, p-LFAs are additionally considerably quicker than gold-standard lab checks, returning ends in solely 20 minutes as an alternative of a number of hours, with comparable or improved sensitivity. The p-LFAs can detect and quantify concentrations of proteins, enabling them to detect bacterial and viral infections in addition to markers of irritation that time to different ailments.
“Plasmonic-fluors are composed of steel nanoparticles that function antennae to tug within the gentle and improve the fluorescence emission of molecular fluorophores, thus making it an ultrabright nanoparticle,” Singamaneni defined.
“Our p-LFAs can choose up even very small concentrations of antibodies and antigens, typical markers of an infection, and provides clinicians clear, fast outcomes with out the necessity for specialised gear. For quantitative testing past the preliminary screening, the identical LFA strip might be scanned with a fluorescence reader, enabling speedy and ultrasensitive colorimetric and fluorometric detection of illness markers with just one check.”
“It is like turning up the quantity on normal color-changing check strips. As a substitute of getting a faint line indicating solely a optimistic or detrimental consequence, the brand new p-LFAs give clearer outcomes with fewer particles, enabling one to maneuver from merely ‘sure or no?’ to precisely ‘how a lot?’ with the help of a cheap, transportable scanner,” stated Jeremiah Morrissey, a analysis professor in anesthesiology within the Division of Medical and Translational Analysis on the College of Medication. Morrissey is a co-author of the brand new research and a long-term collaborator with the Singamaneni lab.
This improved testing functionality has apparent advantages for a inhabitants now all too accustomed to the necessity for fast and dependable check outcomes and the chance of false negatives.
“After we took on this drawback in 2019, we thought our greatest problem can be getting an ample variety of samples from sick folks,” Genin recalled. “The place on Earth may we discover a huge set of samples from sufferers whose signs had been fastidiously documented and whose analysis was verified by sluggish and costly PCR checks?” In a matter of months, COVID-19 would erase that impediment whereas introducing a complete host of latest challenges and alternatives.
“The pandemic was a giant shift for us, prefer it was for everybody,” stated first writer Rohit Gupta, who labored on the p-LFA research as a graduate scholar in Singamaneni’s lab and is now a senior scientist at Pfizer. “We needed to transfer away from our authentic give attention to distinguishing viruses from micro organism, but it surely turned out to be a possibility to do sensible science with actual stakes. We had been working with epidemiologists to get samples for testing, with diagnosticians to check our check to what was out there, and with clinicians to achieve insights into the actual wants for affected person care.”
Enter from your complete collaboration helped Gupta and Singamaneni refine the design of the p-LFAs, which finally achieved 95% scientific sensitivity and 100% specificity for SARS-CoV-2 antibodies and antigens. Genin described the outcomes as beautiful.
“We did not comprehend it was going to work so nicely,” he stated. “We knew it will be good, however we did not know this $1 check with a $300 readout gadget can be so significantly better—10 occasions higher—than state-of-the-art that all of us used in the course of the COVID pandemic.”
Now that they’ve confirmed p-LFAs can outperform normal lab checks in sensitivity, velocity, comfort and price for one illness, the crew is seeking to develop new functions for the know-how, together with returning to their authentic purpose of figuring out bacterial versus viral infections and getting their diagnostic software into the palms of physicians all over the world.
The p-LFA know-how has been licensed to Auragent Bioscience LLC by Washington College’s Workplace of Know-how Administration. Singamaneni and Morrissey are among the many co-founders of Auragent, a WashU startup.
“We count on to have p-LFAs commercially out there within the subsequent one to 2 years,” Singamaneni stated. “Proper now, we’re engaged on bettering our transportable scanner know-how, which provides a extra delicate, fluorescent studying functionality to the check strips along with the colour change that may be seen with the bare eye. We expect we will get that price down to some extent the place it is accessible to rural clinics within the U.S. and overseas, which was one in all our authentic objectives.”
“We’re additionally excited concerning the potential to detect many extra ailments than COVID, presumably utilizing a pores and skin patch that may take a painless pattern,” Singamaneni added. “This know-how has the potential to detect any variety of ailments, starting from STIs to respiratory infections and extra, in addition to cytokines indicative of irritation seen in circumstances resembling rheumatoid arthritis and sepsis.”
Extra data:
Rohit Gupta et al, Ultrasensitive lateral-flow assays through plasmonically lively antibody-conjugated fluorescent nanoparticles, Nature Biomedical Engineering (2023). DOI: 10.1038/s41551-022-01001-1
Offered by
Washington College in St. Louis
Quotation:
New diagnostic check is 1,000 occasions extra delicate than typical checks (2023, February 9)
retrieved 9 February 2023
from https://phys.org/information/2023-02-diagnostic-sensitive-conventional.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.