Synthesis and characterization of pLMOFePt-TGO
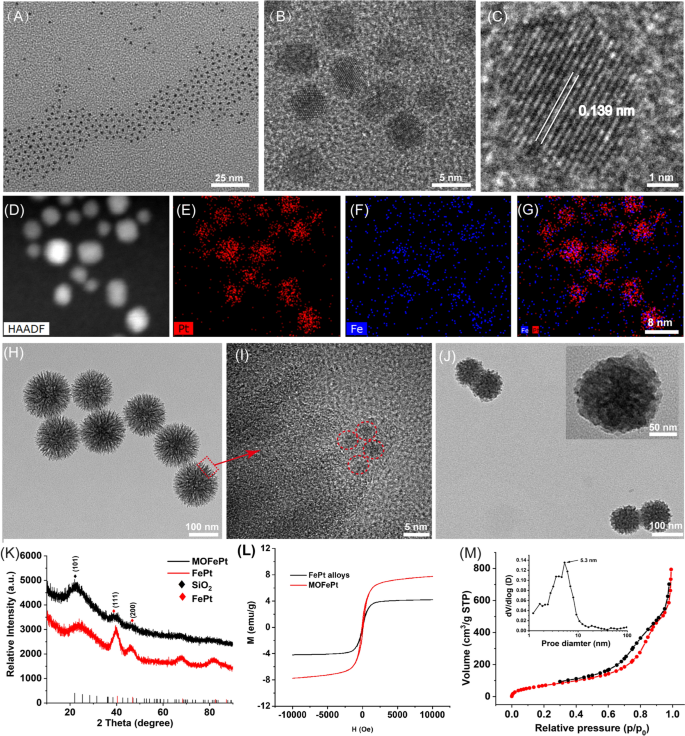
Ultrasmall FePt alloys have been synthesized by a modified thermal decomposition technique. Transmission electron microscope (TEM) photos confirmed that the FePt alloys have been 2–4 nm in dimension and had a slim dimension distribution (Fig. 1A). Excessive-resolution TEM (HRTEM) photos confirmed clear lattice fringes with an interplanar spacing of 0.139 nm, equivalent to the (220) airplane of the FePt alloy (Fig. 1B, C). Elemental mapping evaluation additional confirmed the presence of Fe and Pt, and the absence of O (Fig. 1D–G). These outcomes confirmed that nanoparticles have been composed of the FePt alloy. Subsequently, the FePt alloys have been adorned into disulfide-bridged mesoporous organosilica to acquire MOFePt. It may be seen in Fig. 1H that the MOFePt alloys had a slim dimension distribution centered round 150 nm. The construction of the MOFePt alloys was that of a dendritic sphere, with plentiful pore channels. HRTEM photos additional revealed that the pore channels have been adorned with giant numbers of FePt alloys, implying the profitable preparation of MOFePt alloys (Fig. 1I). Hydrodynamic dimension characterization revealed that whereas the FePt alloy had a slim dimension distribution of roughly 2 nm, the MOFePt alloys have been a lot bigger, roughly 100 nm (Further file 1: Fig. S1A), according to TEM observations.
A–C TEM photos of FePt alloys at completely different magnifications. D–G Elemental mapping photos of FePt alloys. H–I TEM photos of MOFePt at completely different magnifications. J TEM photos of pLMOFePt-TGO. Ok XRD spectra and L, M–H curves of FePt alloy and MOFePt. M N2 adsorption/desorption isotherms of MOFePt
Subsequently, the crystal construction of particles was characterised through X-ray diffraction (XRD). The XRD sample (Fig. 1Ok) reveals peaks at 23.37°, 40.31°, and 46.92°, equivalent to the (101), (111), and (200) planes, respectively, of the FePt alloys (PDF#29-0717). The XRD patterns of MOFePt alloys additionally present vital diffraction peaks at 23.37°, 40.31°, and 46.92°, according to the FePt alloy. Nonetheless, the depth of those peaks in MOFePt was considerably decrease than that of the pure FePt alloy. This may very well be attributed to the shielding impact of MONs. This end result additional proved that the FePt alloys have been efficiently built-in into MON. Area-dependent magnetization (M-H) curves present that the saturated magnetization of MOFePt was 5.28 emu/g, whereas that of FePt alloys was solely 41.72 emu/g (Fig. 1L). This was as a result of the aggregation of FePt alloys in of MON elevated the magnetization of MOFePt. Notably, the FePt and the MOFePt alloys confirmed the standard superparamagnetic habits with out magnetic hysteresis, which could be anticipated to reinforce proton trade and enhance distinction throughout MRI. All these outcomes confirmed that MOFePt was synthesized efficiently as supposed, with acceptable construction and morphology.
N2 adsorption-desorption isotherms indicated that the MOFePt alloys had a definite mesoporous construction and a excessive particular floor space (272.95 m2/g). These nanoparticles have pore sizes primarily within the vary of two–10 nm and a mean pore dimension of roughly 5.3 nm (Fig. 1M). These traits make the MOFePt alloys well-suited to perform as drug carriers. TAM and GOx-loaded MOFePt alloys have been encapsulated by PDGFB-labeled liposomes to manufacture the pLMOFePt-TGO theranostic agent. The hydrodynamic dimension of pLMOFePt-TGO elevated from 150 nm as measured for MOFePt by themselves to roughly 220 nm after encapsulation (Further file 1: Fig. S1A). Furthermore, after the nanoparticle dispersions in varied media have been left undisturbed for twenty-four h, no vital change was noticed within the hydrodynamic dimension of pLMOFePt-TGO nanoparticles, indicating glorious colloidal stability (Further file 1: Figs. S1B, S2). The TEM picture (Fig. 1J) reveals the presence of a definite membrane on the floor of the pLMOFePt-TGO. The complete XPS spectrum of pLMOFePt-TGO introduced in Further file 1: Fig. S3 signifies the presence of the weather Fe, Pt, P, S, Si, C, and O. The high-resolution XPS spectra of pLMOFePt-TGO present Fe 2p peaks at 706.7 eV, and Pt 4f peaks at 74.1 eV and 70.4 eV, indicating the presence of zero-valent Fe and Pt. Whereas, Fe(II) and Fe(III) peaks have been additionally noticed at 710.1 eV and 713.3 eV respectively, they may very well be attributed to some floor oxidation of the FePt alloys. As well as, S2p peaks could be seen at 163.4 eV, equivalent to the binding power of the S–S bonded silicone. These outcomes proved that pLMOFePt-TGO was efficiently fabricated. As well as, the ζ potential of pLMOFePt-TGO was seen to be decrease than that of MON and MOFePt, additional verifying its profitable fabrication (Further file 1: Fig. S4). Fourier rework infrared spectroscopy (FT-IR) characterization of pLMOFePt-TGO confirmed absorption bands at 1400–1600 cm−1, 3500 cm−1 (benzene ring, R-NH2, TAM), and 2850–2930 cm−1 (–CH2, PEG-PDGFB) (Further file 1: Fig. S5A). Moreover, TGA outcomes revealed that the loading charge of TAM in MOFePt-T was ~ 5.07% w/w (Further file 1: Fig. S5B). Subsequently, based mostly on UV customary curve of GOx and ICP evaluation, the loading charge of GOx and FePt in pLMOFePt-TGO have been 8.94% and a couple of.68%, respectively (Further file 1: Fig. S5C, D).
Mobile uptake and biodistribution of nanoplatforms
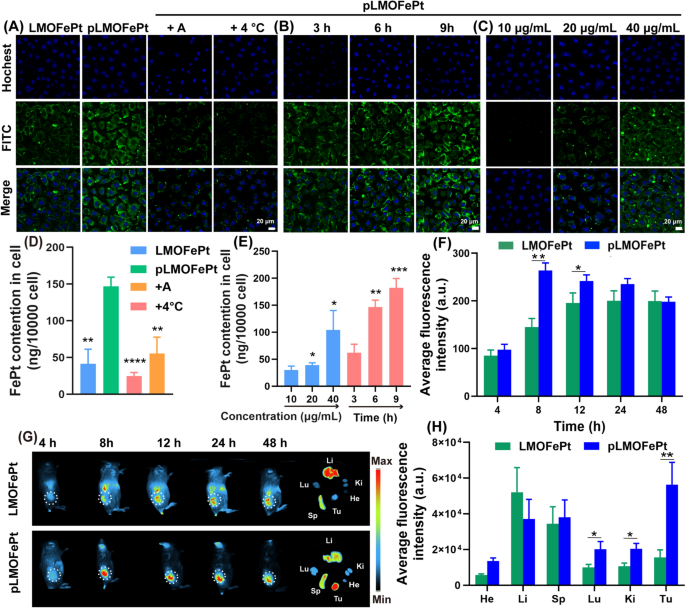
The platelet-derived development issue receptor (PDGFR) pathway is a crucial signaling community for the traditional growth of mesenchymal cells [18]. Overexpression of the PDGF-β receptor is likely one of the widespread options of assorted tumors that embody breast most cancers, cervical most cancers, endometrial most cancers, gastric most cancers, and ovarian most cancers [19,20,21]. Our earlier work has proven that PDGFB ligands could be utilized very successfully to focus on breast cancer-affected tissues [22]. Herein, we noticed the internalization of pLMOFePt to evaluate its focusing on capability through confocal laser microscopy (CLSM) and inductively coupled plasma-mass spectrometry (ICP-MS). LMOFePt and pLMOFePt have been labeled with FITC to point the placement of the nanoparticles throughout the tumor cells. As proven in Fig. 2A, the inexperienced fluorescence depth from MCF-7 cells incubated with pLMOFePt was considerably stronger than these incubated with LMOFePt, indicating that PDGFB has a superb capability to preferentially goal MCF-7 cells. Remarkably, the inexperienced fluorescence of MCF-7 cells handled with pLMOFePt decreased considerably and was nearly negligible within the presence of amiloride (an inhibitor of macropinocytosis), or at a low temperature (4 °C), suggesting that the internalization strategy of pLMOFePt was energy-mediated macropinocytosis. To additional confirm our findings, we used ICP-MS to quantitatively decide the FePt content material in MCF-7 cells cultured with LMOFePt or pLMOFePt. The FePt content material in pLMOFePt-treated MCF-7 cells was nearly threefold that in LMOFePt-treated cells, additional corroborating the superb tumor selectivity of pLMOFePt. Furthermore, mobile FePt content material was noticed to have decreased remarkably after co-incubation with amiloride or at a low temperature (Fig. 2D), which was according to CLSM observations. As well as, the fluorescence sign depth of pLMOFePt-treated MCF-7 cells steadily elevated with a rise in incubation time or dosage, revealing the mobile uptake of pLMOFePt was time- and dosage-dependent (Fig. 2B, C). Equally, the change of nanoparticles quantitative end result as decided by ICP-MS was according to CLSM observations (Fig. 2E). As well as, the internalization of pLMOFePt was additionally investigated in MCF-7 cells by way of bio-TEM remark (Further file 1: Fig. S6C, D). Ample pLMOFePt particles have been noticed in MCF-7 cells and a part of pLMOFePt particles have been vital degraded. Taken collectively, these findings assist that PDGFB might improve the probability and the speed of MCF-7 cells taking in pLMOFePt by endocytosis.
CLSM remark for internalization course of: (A) handled with LMOFePt, pLMOFePt, pLMOFePt + amiloride, and pLMOFePt + 4℃ on the dosage of 40 μg/mL; (B) handled with pLMOFePt for various time on the dosage of 40 μg/mL; (C) handled with completely different concentrations of pLMOFePt for 4 h. ICP-MS quantitative evaluation for internalization course of: (D) handled with LMOFePt, pLMOFePt, pLMOFePt + amiloride, and pLMOFePt + 4℃ on the dosage of 40 μg/mL; (E) handled with completely different concentrations of pLMOFePt and pLMOFePt for various time on the dosage of 40 μg/mL (n=3, imply±SEM, *Means p < 0.05, **Means p < 0.01, ***Means p < 0.001). (F) Fluorescent depth and (G) Fluorescent photos of 4T1 tumor-bearing mice after injection of Cy7-labeled LMOFePt or Cy7-labeled pLMOFePt on the dosage of 10 mg/kg. (H) Fluorescent depth of main organs and tumors in 4T1 tumor-bearing mice after injection Cy7-labeled LMOFePt or pLMOFePt on the dosage of 10 mg/kg for 48 h
We additional explored the biodistribution and tumor-targeted accumulation of pLMOFePt in vivo. A 4T1 tumor-bearing mouse mannequin was constructed by direct subcutaneous injection of 4T1 cells. After a few week, 4T1 tumor-bearing mice have been monitored by an animal imaging system after intravenous injection (I.V.) of LMOFePt-Cy7 and pLMOFePt-Cy7. The fluorescence depth of the tumor in mice injected with pLMOFePt-Cy7 steadily elevated with time from 4 to 24 h, after which steadily decreased. It may be seen in Fig. 2F, that the tumor sign in mice handled with pLMOFePt-Cy7 reached the utmost after 8 h and was considerably stronger than that in mice handled with LMOFePt-Cy7. Determine 2G visually reveals this phenomenon. These mice have been subsequently euthanized, and the key organs and tumors have been excised and noticed utilizing an animal imaging system. As proven in Fig. 2H, the fluorescence depth of the tumor in mice handled with pLMOFePt-Cy7 was greater than 3.6-fold that in mice handled with LMOFePt-Cy7, thus corroborating PDGFB-mediated tumor-targeting functionality in vivo. The biodistribution examine indicated that each LMOFePt-Cy7 and pLMOFePt-Cy7 primarily amassed within the liver and the spleen. Further file 1: Fig. S7A reveals that pLMOFePt-Cy7 had an extended blood retention time in comparison with FePt alloys by themselves. This end result demonstrated that our technique for prolonging the blood circulation time of FePt alloys was profitable, which might enable extra time for the drug to build up within the tumor, thereby enhancing the anti-tumor exercise of this nanoplatform.
TME-dependent degradation and Fenton catalysis traits of MOFePt
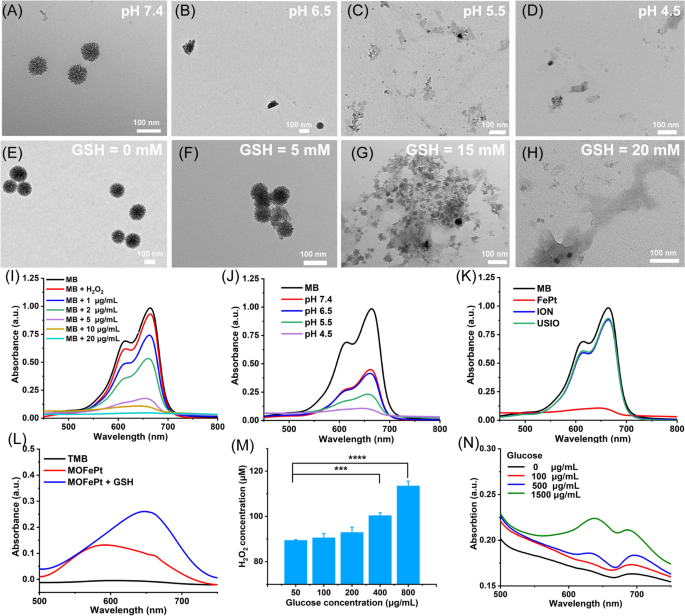
MOFePt alloys have been uncovered to phosphate buffer options with completely different pH values to simulate regular physiological circumstances and TME. As proven within the TEM photos, the construction of MOFePt degraded considerably in low pH circumstances (Fig. 3A–D). Quite a few free FePt alloys have been noticed round collapsed fragments after publicity to a pH of 4.5. As well as, we additionally investigated the biodegradability of MOFePt in response to GSH due to the opportunity of the disulfide bonds within the MONs framework being cleaved by decreased GSH. As anticipated, the degradation of the MOFePt framework was seen to extend in severity with growing GSH focus (Fig. 3E–H). Together with the degradation, FePt alloys have been steadily launched from MOFePt and the discharge habits reveals pH-dependent relation (Further file 1: Fig. S6B). The above outcomes present that the degradation of MOFePt was certainly depending on each the pH in addition to the GSH focus. This helps our proposed technique to attain tumor-specific theranostics utilizing the pLMOFePt-TGO nanoplatform that will get selectively activated in TMEs.
TEM photos of MOFePt degradation at varied (A-D) pH values and (E-H) GSH concentrations for 12 h. Degradation curves of MB answer handled with FePt alloy and H2O2 (10 mM): (I) completely different concentrations of FePt alloys; (J) completely different pH buffers (FePt: 5 μg/mL); (Ok) completely different samples (FePt: 10 μg/mL). (L) TMB coloration assay of MOFePt at absence and presence of GSH (5 mM). (M) H2O2 manufacturing of glucose answer after pLMOFePt-TGO (20 μg/mL) therapy. (N) TMB coloration assay of glucose answer handled with pLMOFePt-TGO (20 μg/mL) (imply±SEM, *Means p < 0.05, **Means p < 0.01, ***Means p < 0.001.
Ferrous ions can catalyze the transformation of hydrogen peroxide (H2O2) into extremely poisonous hydroxyl radicals (·OH) through Fenton catalysis [23]. We subsequent explored whether or not the FePt alloys launched from MOFePt might catalyze the Fenton response. The degradation of methylene blue (MB) was used to evaluate the diploma of ·OH manufacturing. First, we investigated the Fenton-catalytic exercise of FePt alloy. As proven in Fig. 3I, J, MB options handled with FePt alloys degraded shortly and confirmed focus and time-dependent variation. As in comparison with conventional ultrasmall iron oxide (USIO) and iron oxide nanoparticles (ION), FePt alloy confirmed a stronger capability to degrade MB, indicating that the FePt alloys generated extra hydroxyl radicals, and thus have been superior Fenton catalysts (Fig. 3Ok). The flexibility of MOFePt to catalyze Fenton reactions and produce ·OH was additionally detected through a 3, 3′, 5, 5′-tetramethylbenzidine (TMB) coloration assay. As proven in Fig. 3L, the UV absorbance of the H2O2 answer handled with MOFePt and 10 mM of GSH at 650 nm was considerably increased than that with out the addition of GSH. This indicated that the speed of MOFePt catalyzed Fenton response is enhanced within the presence of GSH and produced extra ·OH in consequence. The flexibility of PLMOFePt-TGO to catalyze the transformation of glucose into gluconic acid and H2O2 was additionally assessed utilizing an H2O2 assay package and pH analyzer (Fig. 3M and Further file 1: Fig. S6A). It may very well be seen that the addition of glucose answer generated vital quantities of H2O2, and the acidity of the answer elevated steadily after pLMOFePt-TGO therapy. Furthermore, the rise in H2O2 manufacturing and acidity have been glucose focus dependent. As well as, the TMB coloration assay indicated the UV absorbance on the wavelength of 650 nm steadily elevated with the rise of glucose concentrations within the presence of pLMOFePt-TGO, indicating the power of pLMOFePt-TGO to provide H2O2 and additional induce ROS manufacturing (Fig. 3N). These outcomes demonstrated that pLMOFePt-TGO has immense potential to enhance the efficacy of CDT by self-supplying H2O2 and enhancing acidity.
Mechanism of enhanced ROS manufacturing in vitro
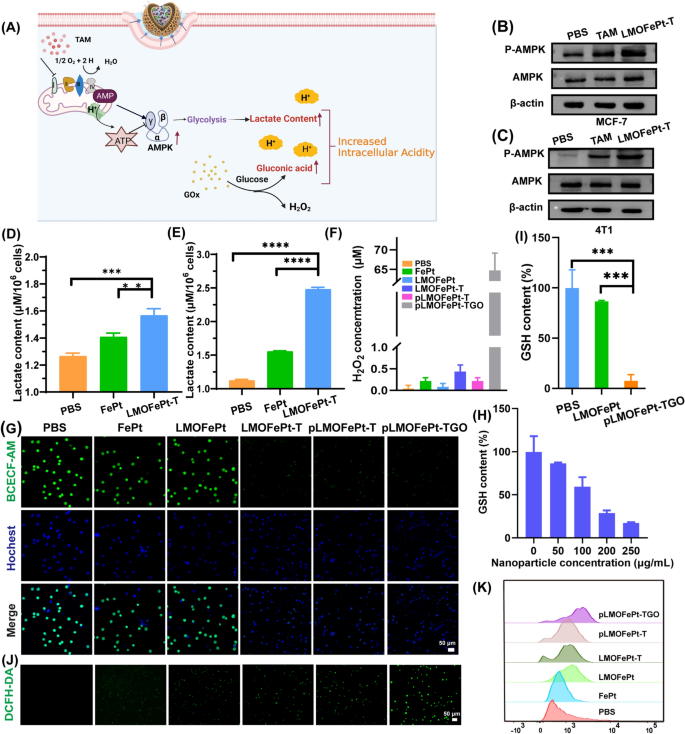
A earlier examine reported that TAM can inhibit the mitochondrial advanced I [24], resulting in a rise within the ratio of adenosine monophosphate (AMP) to adenosine triphosphate (ATP), thereby triggering the AMP-activated protein kinase (AMPK) signaling pathway [25], which is the key regulator of mobile power homeostasis (Fig. 4A). AMPK can promote glycolysis, leading to mobile lactate accumulation [16]. To confirm the glycolysis course of, the expressions of AMPK and p-AMPK in MCF-7 cells and 4T1 cells have been analyzed by western blotting after TAM and pLMOFePt-TG therapy. It may very well be seen that the expression of p-AMPK in 4T1 and MCF-7 cells elevated considerably in TAM and pLMOFePt-T handled teams (Fig. 4B, C). pLMOFePt-T was noticed to have a stronger capability to induce p-AMPK expression than free TAM. These findings supplied strong proof in assist of the speculation that pLMOFePt-T can result in impaired mitochondrial oxidative phosphorylation. Intracellular lactate accumulation was measured utilizing a lactate detection package. As anticipated, the lactate content material of the pLMOFePt-TGO handled cells was considerably increased than these handled with FePt alloys simply by themselves in addition to these handled with simply PBS, notably in hypermetabolic 4T1 tumor cells (Fig. 4D, E). The acidity of MCF-7 cells handled with completely different samples utilizing a pH fluorescent probe 2‘,7‘-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, an acetoxy-methyl ester (BCECF-AM) whose inexperienced fluorescence weakens in acidic circumstances. The inexperienced fluorescence depth of MCF-7 cells handled with FePt alloy by themselves in addition to that of cells handled with LMOFePt was seen to be just like that of the MCF-7 cells from the management group. Nonetheless, the fluorescence depth of the cells handled with LMOFePt-T and pLMOFePt-T was considerably weaker than the management group, with pLMOFePt-T handled cells exhibiting this impact to a larger diploma than the cells handled with LMOFePt-T with out PDGFB labeling. These outcomes proved that pLMOFePt-T might successfully improve mobile acidity through the glycolysis course of. As well as, the acidity of MCF-7 cells handled with pLMOFePt-TGO was increased than these handled with pLMOFePt-T, which may very well be attributed to the synergistic actions of GOx and TAM for enhancing mobile acidity (Fig. 4G). Subsequently, the mobile pH values have been quantitatively analyzed through linear becoming of fluorescence depth (Further file 1: Fig. S7B, C). After therapy with pLMOFePt-TGO, the pH values of the cytoplasm in MCF-7 cells have been round 4.0, which was considerably decrease than that within the untreated most cancers cells. Whereas the restricted H2O2 content material in tumor areas can limit the efficacy of the Fenton reactions [26, 27], GOx can convert intracellular glucose to provide gluconic acid and H2O2, thus growing the acidity domestically [28]. This led us to hypothesize that the upregulation of lactate and gluconic acid and the supplementation of H2O2 in cells would dramatically improve CDT efficacy. We first investigated intracellular H2O2 ranges after completely different pattern remedies. As in comparison with the cells in different teams, the H2O2 content material of MCF-7 cells handled with pLMOFePt-TGO was considerably increased, indicating that pLMOFePt-TGO successfully elevated endogenous H2O2 focus in most cancers cells (Fig. 4F). Subsequently, we concluded that pLMOFePt-TGO provided plentiful endogenous H2O2 in addition to enhanced mobile acidity by the synergistic motion of TAM-mediated non-oxygen glycolysis and the oxidation of glucose by GOx, considerably enhancing the Fenton catalytic exercise and growing the anti-cancer efficacy of CDT. It’s well-known that GSH is an antioxidant that reduces the ROS focus and weakens the anti-cancer efficacy of CDT. Our outcomes present that pLMOFePt-TGO might successfully sort out this problem by consuming GSH due to the presence of disulfide bonds. We investigated mobile GSH ranges after completely different pattern remedies. As proven in Fig. 4H, whereas LMOFePt considerably decreased mobile GSH, pLMOFePt-TGO confirmed the strongest capability to scale back mobile GSH (Fig. 4I). Subsequently, we additional investigated ROS era in MCF-7 most cancers cells utilizing a 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) probe. As proven in Fig. 4J, the inexperienced fluorescence of MCF-7 cells handled with FePt or LMOFePt alone elevated barely, whereas cells handled with LMOFePt-T and pLMOFePt-T confirmed a major improve within the depth of inexperienced fluorescence. Notably, the pLMOFePt-TGO-treated cells confirmed the strongest inexperienced fluorescence, indicating a dramatic improve in mobile ROS manufacturing. In the meantime, these outcomes have been additionally additional confirmed by stream cytometry (Fig. 4Ok). These outcomes demonstrated that pLMOFePt-TGO enhanced endogenous H2O2 ranges and intracellular acidity in tumor cells, in addition to consumed intracellular GSH, and in consequence, considerably amplified the Fenton response, producing plentiful ROS.
A Schematic diagram of mechanism of intracellular acidity enhancement. Dedication of intracellular p-AMPK protein ranges in B MCF-7 cells and C 4T1 cells by western blotting assay. Intracellular lactate content material of D MCF-7 and E 4T1 cells incubated with PBS, FePt alloys, and LMOFePt-T at 5 μg/mL (equal Fe) for 12 h. F H2O2 content material of MCF-7 cells handled with completely different samples on the focus of 5 μg/mL (equal Fe) for 12 h. G CLSM remark for the acidity of MCF-7 cells stained by BCECF-AM after completely different samples on the focus of 5 μg/mL (equal Fe) for 12 h. H GSH degree of MCF-7 cells incubated with completely different concentrations of pLMOFePt-TGO. I GSH degree of MCF-7 cells incubated with LMOFePt-TGO and pLMOFePt-TGO on the focus of 20 μg/mL for twenty-four h. ROS manufacturing of MCF-7 cells stained by DCFH-DA after therapy with completely different samples for 4 h: J CLSM remark; Ok consultant stream cytometric evaluation. For D, E and I, knowledge are means ± SD, n = 3, *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001
In vitro antitumor mechanism examine
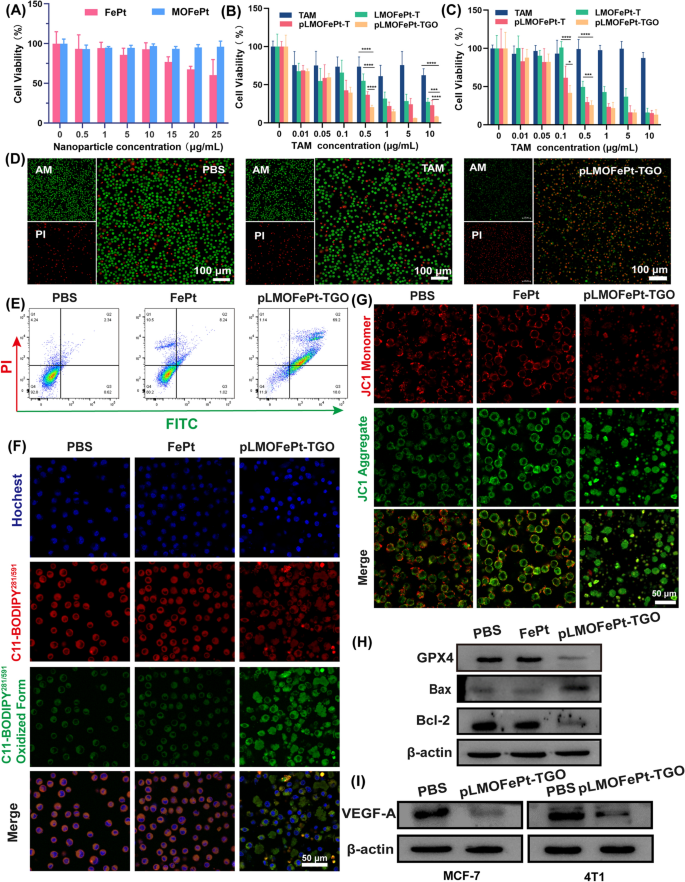
The viability of cells was investigated by MTT assay. As in comparison with FePt alone, MOFePt didn’t trigger a lower in cell viability, exhibiting decrease cytotoxicity to the THLE-3 cells. This end result reveals that the MOFePt nanocarriers have glorious biocompatibility (Fig. 5A). As well as, the anti-cancer efficacy of pMOFePt-TGO towards MCF-7 and 4T1 cells was assessed at completely different dosages. As proven in Fig. 5B, C, all samples confirmed a concentration-dependent variation in cell viability. Though TAM confirmed a sure diploma of inhibition on MC-7 cells, it didn’t present any toxicity towards 4T1 cells. This was as a result of TAM solely selectively focused estrogen receptor-positive (ER+) cells. Notably, whereas each LMOFePt-T and pLMOFePt-T exhibited a larger diploma of inhibition on the viability of MCF-7 and 4T1 cells as in comparison with TAM, the cytotoxicity of pLMOFePt-T was stronger than that of LMOFePt-T with out PDGFB labeling. These outcomes demonstrated that the anti-cancer exercise pLMOFePt-T was unbiased of the presence or absence of estrogen receptors and was strongly related to glycolysis-enhanced CDT. As anticipated, pLMOFePt-TGO therapy exhibited the strongest anti-cancer inhibitory impact as in comparison with the opposite teams, indicating that the GOx-mediated hunger remedy additional enhanced the efficacy of the ant-cancer CDT utilizing pLMOFePt-TGO. With a purpose to affirm the presence of ferroptosis, we explored the viability of MCF-7 cells handled with pLMOFePt-TGO on the absence and presence of iron ion chelate (DFOM) or ferroptotic inhibitor (Fer-1). As proven in Further file 1: Fig. S8, the viability of MCF-7 cells considerably recovered after DFOM and Fer-1 therapy, additional indicating the presence of ferroptosis. As well as, we additionally discovered that using NAC and apoptotic inhibitor additionally considerably recovered the viability of MCF-7 cells, indicating the presence of ROS-induced cell dying and apoptosis. These outcomes demonstrated that pLMOFePt-TGO-mediated cell dying is derived from ferroptosis and apoptosis. The dwell/lifeless cell staining assay additional confirmed that the anti-cancer exercise of pLMOFePt was essentially the most potent among the many remedies examined on this examine tumor cells (Fig. 5D).
Viability of A THLE-3, B MCF-7, and C 4T1 cells handled with completely different samples. D Reside/lifeless staining of MCF-7 cells handled completely different samples (equal TAM: 5 µg/mL). E Movement cytometry evaluation for the apoptosis of MCF-7 cells on the completely different remedies (equal FePt: 5 µg/mL). F CLSM remark for the LPO of MCF-7 cells handled with completely different samples (equal FePt: 5 µg/mL). G CLSM remark for mitochondria injury of MCF-7 cells handled with completely different samples (equal FePt: 5 µg/mL). H–I Western blot evaluation for the protein expression of Bcl-2, Bax, GPX4, and VEGF-A at completely different pattern remedies (equal FePt: 5 µg/mL). For B and C, knowledge are means ± SD. n = 5, *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001
The quantitative stream cytometric outcomes proven in Fig. 5E indicated that the apoptotic/necrotic proportion of the cells handled with pLMOFePt-TGO was 88.34%. Iron ion-mediated Fenton response induces extreme ROS manufacturing in cells, which might set off ferroptosis [29]. The expression of glutathione peroxidase 4 (GPX4) as a ferroptotic marker in MCF-7 cells was assessed after varied remedies by western blotting. Important downregulation of GPX4 was noticed within the pLMOFePt-TGO group, suggesting that pLMOFePt-TGO induced ferroptosis (Fig. 5H). To confirm pLMOFePt-TGO-induced ferroptosis, we additional detected the intracellular lipid hydroperoxides (LPO) utilizing the BPDIPY probe through CLSM remark. As proven in Fig. 5F and Further file 1: Fig. S9B, the inexperienced fluorescence of MCF-7 and 4T1 cells incubated with the pLMOFePt-TGO was considerably stronger than these of the cells incubated in PBS or FePt alloy, clearly supporting the presence of LPO in cells. Therefore, we concluded that the plentiful ROS generated by pLMOFePt-TGO elevated the LPO focus in most cancers cells, and induced ferroptosis in them. As well as, extreme ROS additionally broken the mitochondria, inducing mitochondria-mediated apoptosis. We evaluated the change of mitochondrial membrane potential (MMP) utilizing the JC-1 probe which modified coloration from pink to inexperienced in cells within the presence of broken mitochondria. As introduced in Fig. 5G and Further file 1: Fig. S9A, MCF-7 and 4T1 most cancers cells incubated with pLMOFePt-TGO confirmed the strongest inexperienced fluorescence and the weakest pink fluorescence as in comparison with different teams, indicating a major discount of MMP. As well as, western blot evaluation revealed the upregulation of Bax expression, and down-regulation of Bcl-2 expression within the pLMOFePt-TGO group, additional confirming the incidence of mitochondria-mediated apoptosis (Fig. 5H). Furthermore, we discovered that the expression of vascular endothelial development issue A (VEGF-A) in cells handled with pLMOFePt-TGO was considerably down-regulated. In addition to, we additionally additional investigated the anti-angiogenesis impact of pLMOFePt-TGO by tube formation assay of C166 endothelial cells. As proven in Further file 1: Fig. S10, pLMOFePt-TGO considerably blocked tube formation of C166 cells, and confirmed concentration-dependent antiangiogenic potential. This end result additional demonstrated glorious anti-angiogenesis of pLMOFePt-TGO. These outcomes demonstrated that the anti-cancer mechanism of pLMOFePt-TGO was additionally concerned in mitochondria-mediated apoptosis and anti-tumor angiogenesis.
In vivo anti-cancer efficacy
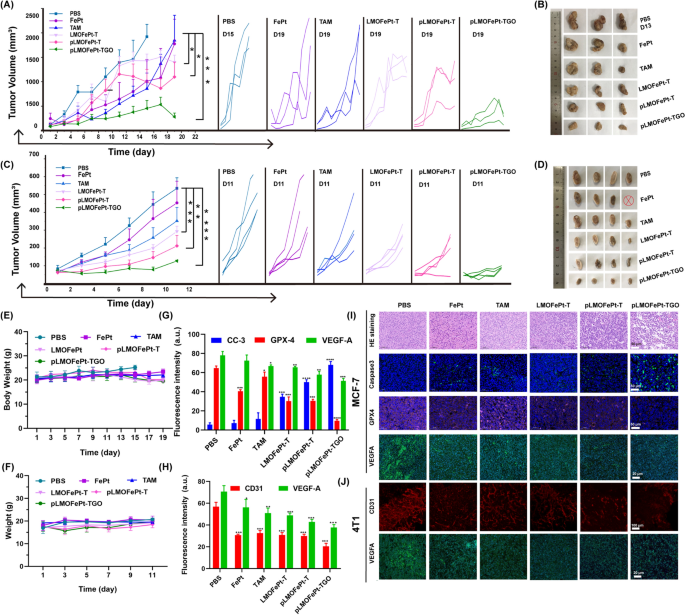
The in vivo anticancer efficacy of pLMOFePt-TGO was investigated by MCF-7 and 4T1 tumor-bearing mice. All animal experiments have been authorized by the Analysis Institute Ethics Committee of Binzhou Medical College and have been performed in accordance with the rules on the use and care of laboratory animals at Binzhou Medical College. The tumor-bearing mice have been intravenously injected with PBS, FePt, TAM, LMOFePt-T, pLMOFePt-T, and pLMOFePt-TGO (2 mg/kg) at two-day intervals. As proven in Fig. 6A, all of the therapy teams confirmed completely different levels of inhibition on MCF-7 tumor development as in comparison with the PBS group. Furthermore, it was discovered that the tumor quantity of PBS-treated MCF-7 tumor-bearing mice elevated to roughly 2000 mm3 in 13 days. The PBS-treated MCF-7 tumor-bearing mice have been euthanized after 13 days, and the tumors have been excised. The tumor quantity of MCF-7 tumor-bearing mice injected with pLMOFePt-TGO was the smallest amongst all of the teams on this examine, indicating it had the strongest anti-cancer exercise. After therapy for 19 days, the mice in different teams have been additionally euthanized, and the tumors have been excised and photographed (Fig. 6B). The pLMOFePt-TGO group had the smallest tumor dimension and least tumor weight. Comparable outcomes have been noticed within the 4T1 tumor mannequin (Fig. 6C, D). This end result demonstrated that the anticancer exercise of pLMOFePt-TGO was unbiased of the expression of estrogen receptors in breast most cancers. Mice in all of the teams had no vital physique weight reduction all through the remedy (Fig. 6E, F). The very important organs and the tumor tissues have been minimize into slices and stained utilizing the hematoxylin and eosin (H&E) and immunofluorescence (IF) staining technique. Furthermore, no deaths of 4T1 and MCF-7 tumor-bearing mice have been famous within the pLMOFePt-TGO group, and the ends in Further file 1: Fig. S11 confirmed no injury in main organs, implying glorious biosafety of pLMOFePt-TGO. As proven in Further file 1: Fig. S12, Fig. 6G, I, the diploma of expression of Ki67 in tumors within the varied therapy teams was discovered to be within the following order: PBS > FePt > TAM > LMOFePt-T > pLMOFePt-T > pLMOFePt-TGO, whereas the diploma of expression of cleaved caspase-3 in tumor tissues was within the reverse order. These outcomes demonstrated that pLMOFePt-TGO had the best apoptosis-inducing capability and permitted the bottom proliferation of tumor cells. As well as, the GPX4 ranges detected within the tumor slices within the varied therapy teams have been the next order: PBS > TAM > FePt > LMOFePt-T > pLMOFePt-T > pLMOFePt-TGO. This end result additional confirmed the concept ferroptosis was induced by pLMOFePt-TGO. Moreover, the pLMOFePt-TGO group had the best proportion of apoptotic cells and the utmost variety of necrotic areas as in comparison with the opposite teams. These outcomes amply demonstrated that pLMOFePt-TGO therapy had the strongest anti-tumor exercise among the many medication examined owing to the synergistic integration of chemotherapy, CDT, and hunger remedy.
Tumor volumes and pictures of MCF-7 tumor and 4T1 tumor A–D bearing mice injected with completely different samples on the injection dosage of two mg/kg, ⊗ represents dying. E Physique weight adjustments of MCF-7 tumor-bearing mice handled with completely different samples. F Physique weight adjustments of 4T1 tumor-bearing mice handled with completely different samples. The corresponding fluorescent quantification evaluation for IF staining photos of G MCF-7 tumor and H 4T1 tumor slices. I IF and H&E staining photos of MCF-7 tumor after therapy. J CD31 entire mount staining and IF photos of 4T1 tumor after therapy. (imply ± SEM, *Means p < 0.05, **Means p < 0.01, ***Means p < 0.001)
PDGFR-β is thought to be overexpressed in most tumor stromal cells and tumor vasculature [30]. Therefore, pLMOFePt-TGO may additionally be anticipated to focus on tumor vasculature and inhibit tumor angiogenesis. Furthermore, it was beforehand reported that TAM contributed to chemotherapy in addition to downregulated VEGFA and induced anti-angiogenesis [31]. We additional analyzed tumor angiogenesis by whole-mount CD31 staining. In Fig. 6J, PBS-treated tumor slices confirmed a dense vascular community, whereas the tumor slices of mice handled with different medicine confirmed vasculature that was considerably much less dense, and tumor tissues from mice handled with pLMOFePt-TGO confirmed most suppression of tumor angiogenesis. This end result proved our speculation that pLMOFePt-TGO might immediately goal tumor vessels and inhibit tumor angiogenesis. The statistical evaluation of CD31 fluorescence sign depth confirmed this conclusion (Fig. 6H). Immunofluorescence (IF) characterization confirmed that the expression of VEGFA was considerably downregulated in tumors handled with pLMOFePt-TGO as in comparison with different teams, indicating that pLMOFePt-TGO additionally inhibited tumor VEGFA expression and triggered tumor anti-angiogenesis (Fig. 6G, H). Subsequently, we concluded that pLMOFePt-TGO confirmed glorious tumor anti-angiogenesis through dual-targeting pathways.
In vivo MRI research
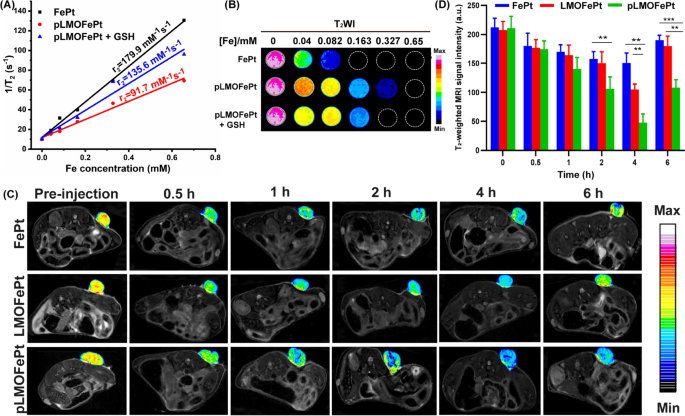
Owing to the presence of the superparamagnetic FePt alloys, pLMOFePt-TGO might have vital ramifications in magnetic resonance imaging (MRI) prognosis. Transverse relaxivity (r2) measurements of FePt and pLMOFePt have been performed utilizing a 7.0 T MRI scanner. The r2 values of FePt alloy and pLMOFePt have been 179.9 mM−1 s−1 and 91.7 mM−1 s−1 respectively (Fig. 7A, B). The r2 worth of pLMOFePt was decrease than that of FePt, which was attributed to the confinement impact of the natural silica framework. Notably, after GSH therapy, the r2 worth of pLMOFePt elevated to 135.6 mM−1 s−1, exhibiting a rest change. This confirmed that pLMOFePt degraded within the TME, releasing FePt alloy, which helped to enhance distinction in T2-weighted MRI scans. The flexibility of pLMOFePt to reinforce MRI distinction was additionally explored utilizing a 4T1 tumor-bearing mouse mannequin. T2-weighted photos of the tumors have been acquired at completely different time intervals (Fig. 7C). The components of the MRI scan equivalent to the tumor in mice injected with pLMOFePt steadily darkened, indicating that pLMOFePt quickly amassed within the tumor. In distinction, the areas within the MRI scans equivalent to the tumors in mice injected with FePt alloys solely darkened barely. This may very well be attributed to the FePt alloys being simply metabolized, thus impeding their accumulation within the tumor as a result of EPR impact. As well as, the adjustments within the sign depth of the tumor area have been decided utilizing MRIcro software program. The minimal T2 sign depth of the tumor area handled with FePt alloys, LMOFePt, and pLMOFePt was noticed to succeed in 150.45, 105.00, and 47.58, respectively (Fig. 7D). Thus, it’s evident that among the many supplies examined, pLMOFePt can be the most effective at enhancing the distinction of the tumor in T2-weighted MRI scans, including to its potential as theranostic for tumors.
A The r2 values B corresponding pseudocolor T2-weighted MR photos of FePt alloys and pLMOFePt with and with out GSH (10 mM) therapy. C T2-weighted MR photos and D corresponding MRI sign adjustments of tumor at axial airplane at post-injection (2 mg/kg) of FePt alloys, LMOFePt, and pLMOFePt (imply ± SEM, *Means p < 0.05, **Means p < 0.01, ***Means p < 0.001)